Antisense LNA GapmeR Premium (5 nmol)
Cat. No. / ID: 339517
Features
- Function by RNase H-dependent degradation of complementary RNA targets
- Provide strand-specific knockdown with no RISC-associated off-target activity
- Active in vivo and in vitro, enabling the analysis RNA function in a wide range of model systems
- Excellent alternative to siRNA for knockdown of mRNA and lncRNA
- Taken up by cells without need for transfection reagents
- Variety of controls support transfection or unassisted uptake protocol optimization
Product Details
Antisense LNA GapmeRs are highly potent, single-stranded antisense oligonucleotides (ASO) for silencing of lncRNA and mRNA in cell cultures and even in animal models. Antisense LNA GapmeR Positive Controls enable optimization of conditions for lipid-based transfection, electroporation or unassisted delivery. The GapmeRs are enhanced with LNA technology and are designed using sophisticated and empirically developed algorithms and offer excellent performance and high success rates.
Need a quote for your research project or would you like to discuss your project with our specialist team? Just contact us!
Performance
Potent knockdown of mRNA or lncRNA
The efficacy of mRNA knockdown using Antisense LNA GapmeRs rivals that of siRNA-based methods (see figure Antisense LNA GapmeRs have higher success rate and potency than siRNAs), providing an excellent alternative for researchers looking for a technique that works independently of RISC and has no miRNA-like, off-target effects.Tool-of-choice for silencing of lncRNA
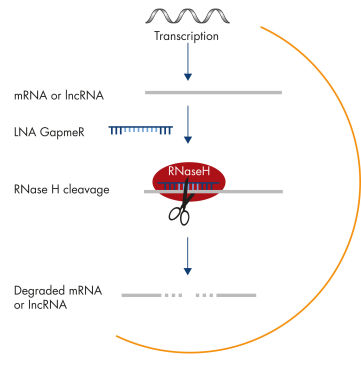
lncRNA loss-of-function studies can be particularly challenging for several reasons. Many lncRNAs are involved in transcriptional regulation by attracting chromatin-modifying enzymes to certain DNA targets. Since they are confined to the nuclear compartment, these lncRNAs are inefficiently targeted by siRNA. In contrast, RNAs retained in the nucleus are particularly sensitive to Antisense LNA GapmeRs, because they share the nuclear compartment with RNase H, the endonuclease responsible for Antisense LNA GapmeR activity (see figure Silencing of mRNA and long non-coding RNA using Antisense LNA GapmeRs). In addition, lncRNAs often derive from transcriptionally complex loci with overlapping sense and antisense transcripts. Strand-specific knockdown is therefore crucial, and this is guaranteed with Antisense LNA GapmeRs, because they are single stranded. Antisense LNA GapmeRs provide effective knockdown of various lncRNAs, regardless of their intracellular localization (see figure Efficient knockdown with Antisense LNA GapmeRs, regardless of RNA target type and subcellular localization).No transfection reagent needed
Antisense LNA GapmeRs are efficiently taken up by cells directly from the culture medium due to their small size and exceptional potency and stability. This makes it possible to achieve potent knockdown of target RNA in many cell lines with unassisted delivery (see figure LNA GapmeRs can be used without a transfection agent), avoiding the cytotoxic effects associated with transfection reagents. Non-assisted uptake does require higher concentrations of the Antisense LNA GapmeR than would be needed with lipid-based transfection, and the knockdown kinetics are slower. Usually, knockdown is observed after only 48 H of culture in the presence of the Antisense LNA GapmeR.Potent positive controls with optimal specificity
Antisense LNA GapmeR Positive Controls are experimentally validated and feature very potent activity against different types of RNA targets expressed in a broad range of cell types. The controls are available for different types of RNA with different subcellular localization (see figure Performance of Antisense LNA GapmeR Positive Controls), making it possible to identify an appropriate control for most applications. Every Antisense LNA GapmeR Positive Control was designed for optimal specificity and was selected based on experiments demonstrating highly potent activity against its intended target.Study RNA function in live animal models
Excellent pharmacokinetic and pharmacodynamic properties of Antisense LNA GapmeRs have been demonstrated in many different tissues and organs. These LNA antisense oligonucleotides are well tolerated and show low toxicity in vivo. In addition, short, high-affinity Antisense LNA GapmeRs are active at lower concentrations than other antisense oligonucleotides. The incorporation of LNA also increases the serum stability of the ASO.Antisense LNA GapmeRs have high potential to penetrate the cell membrane barrier and successfully interact with intracellular and even nuclear-retained targets. They also provide effective and long-lasting knockdown of mRNA and lncRNA in a broad range of tissues in live animal models. Plus, the workflow is easier, because specific formulation using liposomes or cationic complexes, for example, is not required for efficient in vivo delivery. See figure Efficient in vivo knockdown with LNA GapmeRs in a broad spectrum of tissues for an example of in vivo knockdown of a highly abundant, nuclear-retained lncRNA.
See figures
 Silencing of mRNA and long non-coding RNA using Antisense LNA GapmeRs.
Silencing of mRNA and long non-coding RNA using Antisense LNA GapmeRs.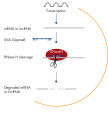 Efficient knockdown with Antisense LNA GapmeRs, regardless of RNA target type and subcellular localization.
Efficient knockdown with Antisense LNA GapmeRs, regardless of RNA target type and subcellular localization. LNA GapmeRs can be used without a transfection agent.
LNA GapmeRs can be used without a transfection agent.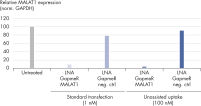 Performance of Antisense LNA GapmeR Positive Controls.
Performance of Antisense LNA GapmeR Positive Controls. Efficient in vivo knockdown with LNA GapmeRs in a broad spectrum of tissues.
Efficient in vivo knockdown with LNA GapmeRs in a broad spectrum of tissues.
Principle
Efficient silencing of mRNA and lncRNA with fewer off-target effects
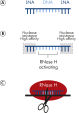
Antisense LNA GapmeRs are powerful tools for protein, mRNA and lncRNA loss-of-function studies. These single-stranded, antisense oligonucleotides (ASOs) catalyze RNase H-dependent degradation of complementary RNA targets. The Antisense LNA GapmeRs are 16 nucleotides long and are enriched with LNA in the flanking regions and DNA in an LNA-free central gap, hence the name "GapmeR" (see figure A unique short, single-stranded antisense design). The LNA-containing flanking regions confer nuclease resistance to the antisense oligo, while also increasing target binding affinity, regardless of the GC content. The central DNA "gap" activates RNase H cleavage of the target RNA upon binding. Antisense LNA GapmeRs have fully modified phosphorothioate (PS) backbones, which ensure exceptional resistance to enzymatic degradation.
Sophisticated design parameters
Antisense LNA GapmeRs are designed using our empirically derived design tool that incorporates more than 20 years of experience with LNA design. For each RNA target, the tool evaluates thousands of possible designs against more than 30 design parameters to identify the Antisense LNA GapmeRs most likely to provide potent and specific target knockdown.
The primary design parameters include the following:
- Optimal target sequence accessibility to ensure high potency. The design tool selects target sequences based on local secondary structure prediction.
- Antisense off-target evaluation. GapmeR sequences are aligned against ENSEMBL to enable selection of the most specific Antisense LNA GapmeRs with minimal off-targets in the spliced and unspliced transcriptomes.
- Optimal oligonucleotide design, including length, Tm, gap size, self-complementarity, LNA positions, etc.
Coverage
Antisense LNA GapmeRs can be designed for any RNA target >80 nucleotides and are available in several different grades, depending on your application:
- Antisense LNA GapmeR Standard: cost-effective LNA GapmeRs in tubes for initial testing of multiple designs using standard cell lines.
- Antisense LNA GapmeR Premium: HPLC-purified LNA GapmeRs with guaranteed purity suited for most cell assays; also available with 5’ or 3’ fluorescent labels.
- Antisense LNA GapmeR in vivo Ready: high-quality, animal-grade LNA GapmeRs recommended for any projects with in vivo testing as the ultimate goal. Also recommended for difficult-to-transfect cell lines, such as B cells, primary cell lines, cells in suspension, etc.
- Antisense LNA GapmeR in vivo Large Scale: high-quality, animal-grade LNA GapmeRs available in a range of scales with various purification and labeling options.
- Antisense LNA GapmeR Custom Plates: Standard LNA GapmeRs delivered in convenient 96-well plate format for screening projects.
Highly purified Antisense LNA GapmeRs for in vivo studies
We also offer higher-purity Antisense LNA GapmeRs for use in in vivo studies. These Antisense LNA GapmeRs have excellent pharmacokinetic and pharmacodynamic properties and offer effective and long-lasting RNA silencing in a broad range of tissues. They are well-tolerated and show low toxicity in vivo, due to the following unique features:
- High target affinity: LNA nucleotides positioned at the extremities increase affinity for the target.
- Excellent stability: the combination of LNA nucleotides and phosphorothioate backbone modifications lead to high stability. The LNA GapmeRs are highly resistant to nucleases and have a long half-life in serum.
- Short length: LNA modifications enable design of short (15–16mer) gapmers with high target affinity. This is much shorter than conventional gapmers, which are typically around 20 nucleotides long. Shorter oligonucleotides are generally taken up much more efficiently via natural cellular uptake mechanisms, so Antisense LNA GapmeRs are highly effective in live animal models. Short oligonucleotides are also less prone lead to toxicity from binding proteins, such as Toll-like receptors and components of the complement system.
- Excellent biodistribution: oligonucleotides with standard phosphodiester backbones are lost into the urine. However, PS-modified oligonucleotides bind with albumin in the bloodstream. This extensive but low-affinity binding helps prevent loss of PS-modified oligonucleotides by renal filtration and facilitates rapid uptake in a broad range of tissues.
| Stage | Design | Screening | in vitro Optimization | in vivo Studies | |
| Requirement |
|
|
|
|
|
| Solution | Online GapmeR design tool | Antisense LNA GapmeR Standard or GapmeR Custom Plates | Antisense LNA GapmeR Premium | Antisense LNA GapmeR in vivo Ready | Antisense LNA GapmeR in vivo Large Scale |
| Product | Cat. no. | Purity | Amount | PS | Standard desalting |
HPLC | Na+ exchange |
Available with 3' and 5' FAM |
| Antisense LNA GapmeR Standard | 339511 339512 |
No purity guarantee | 5 nmol 15 nmol |
yes | yes | no | no | yes |
| Antisense LNA GapmeRPremium | 339517 339518 |
≥75% | 5 nmol 15 nmol |
yes | no | yes | no | yes |
| Antisense LNA GapmeR in vivo Ready | 339523 339524 |
≥75% | 5 nmol 15 nmol |
yes | no | yes | yes | yes |
| Antisense LNA GapmeR in vivo Large Scale | 339532 | Custom | Custom, from 5 mg to 1 kg |
yes | yes/no | yes/no | yes/no | yes |
See figures
Procedure
Thein vivo grade of Antisense LNA GapmeRs is available with fluorescein or other custom modifications. They are delivered dried down in Na salt, ready to be dissolved in PBS. If needed, they can be delivered in the quality required and with the necessary documentation for preclinical toxicity studies and human trials. After resuspension, these GapmeRs can be directly injected systemically with no need for expensive formulation in nanoparticles or liposomes. LNA GapmeRs can be administered via various routes (subcutaneous, intravenous, intraperitoneal, etc.). For more information, refer to the in vivo guidelines.
Applications
When delivered effectively to cell cultures, Antisense LNA GapmeR Positive Controls are certain to provide very significant knockdown of their RNA targets, so they are very useful for optimizing conditions for lipid-based transfection or electroporation. They can also be used to test the susceptibility of a particular cell line to unassisted uptake.
RNA function in animal models can be inferred by studying the biological consequences of RNA silencing with Antisense LNA GapmeRs. Such studies have revealed the important role played by lncRNAs in several human diseases.
Supporting data and figures
Silencing of mRNA and long non-coding RNA using Antisense LNA GapmeRs.