QIAprep Spin Miniprep Kit
Zur Aufreinigung von bis zu 20 µg molekularbiologischer Plasmid-DNA
Zur Aufreinigung von bis zu 20 µg molekularbiologischer Plasmid-DNA
Cat. No. / ID: 27104
Das QIAprep Spin Miniprep Kit ist für die Isolierung von hochreiner Plasmid- oder Cosmid-DNA konzipiert und liefert Ausbeuten von bis zu 20 µg für verschiedene molekularbiologische Anwendungen wie Sequenzierung und Klonierung. Höhere Ausbeuten (bis zu 30 µg) können mit dem Zusatzprotokoll für hohe Ausbeuten erzielt werden. Das Kit ist auf dem QIAcube Connect automatisierbar.
Möchten Sie das QIAprep Spin Miniprep Kit zum ersten Mal ausprobieren? Fordern Sie ein Angebot für ein Testkit an.
Für optimale Ergebnisse empfehlen wir, dieses Kit mit dem QIAvac 24 Plus-System zu verwenden.
Als nachhaltigere Alternative bieten wir das QIAwave Plasmid Miniprep Kit an. Dieses Kit reduziert den Verbrauch von Plastik um bis zu 22 % und von Karton um bis zu 14 %. Es enthält Abfallröhrchen aus 100 % recyceltem Kunststoff, die während des gesamten Verfahrens wiederverwendet werden können. Die QIAwave-Puffer liegen als Konzentrat vor und senken den Kunststoffverbrauch pro Flasche um bis zu 93 %. Trotz des optischen Unterschieds ist auch das QIAwave Kit benutzerfreundlich und in Chemie und Leistung identisch mit dem Standard-Kit.
Bitte beachten Sie, dass Sie für die Lagerung der rekonstituierten Puffer sterile Glasflaschen benötigen.
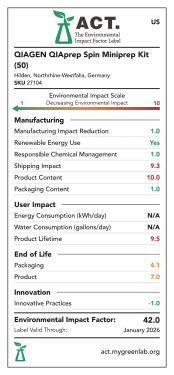
In Zusammenarbeit mit My Green Lab haben wir auch die Umweltauswirkungen des QIAprep Spin Miniprep Kit (50/250) und des QIAwave Plasmid Miniprep Kit (50/250) bewertet. Die ACT-Labels von My Green Lab wurden dafür entwickelt, Produkte nach verschiedenen Nachhaltigkeitskriterien zu bewerten und einzustufen. Dazu gehören:
• Herstellung
• Verantwortungsvoller Umgang mit Chemikalien
• Nachhaltige Inhalte in Produkten und Verpackungsmaterialien
• Entsorgung der Verpackung am Ende des Lebenszyklus
Die Produkte werden von 1 bis 10 bewertet, mit Ausnahme von Energie- und Wasserverbrauch, die mit 1 Punkt pro kWh bzw. Gallone (3,785 Liter) bewertet werden. Eine niedrige Punktzahl bedeutet eine geringere Umweltauswirkung – (siehe Abbildungen „„QIAprep Spin Miniprep Kit ACT-Umweltverträglichkeitslabel USA ( 50/250), EU (50/250) und UK (50/250)““ und “QIAwave Plasmid Miniprep Kit ACT-Umweltverträglichkeitslabel USA ( 50/ 250), EU ( 50/ 250) und UK (50/ 250).“
 QIAwave Plasmid Miniprep Kit (50) ACT-Umweltverträglichkeitslabel USA.
QIAwave Plasmid Miniprep Kit (50) ACT-Umweltverträglichkeitslabel USA. QIAwave Plasmid Miniprep Kit (250) ACT-Umweltverträglichkeitslabel USA.
QIAwave Plasmid Miniprep Kit (250) ACT-Umweltverträglichkeitslabel USA. QIAwave Plasmid Miniprep Kit (50) ACT-Umweltverträglichkeitslabel EU.
QIAwave Plasmid Miniprep Kit (50) ACT-Umweltverträglichkeitslabel EU.  QIAwave Plasmid Miniprep Kit (250) ACT-Umweltverträglichkeitslabel EU.
QIAwave Plasmid Miniprep Kit (250) ACT-Umweltverträglichkeitslabel EU. QIAwave Plasmid Miniprep Kit (250) ACT-Umweltverträglichkeitslabel UK.
QIAwave Plasmid Miniprep Kit (250) ACT-Umweltverträglichkeitslabel UK.
Das QIAprep Spin Miniprep Kit oder QIAwave Plasmid Miniprep Kit ermöglicht die Aufreinigung von bis zu 20 µg Plasmid-DNA oder Cosmid-DNA in molekularbiologischer Qualität für den Einsatz in molekularbiologischen Routineanwendungen wie PCR, Sequenzierung und Klonierung. Die vielseitigen QIAprep 2.0 Spin Columns können entweder in Mikrozentrifugen, auf Vakuumverteilern oder im QIAcube verwendet werden (siehe Abbildungen „Handhabungsoptionen A, B und C der QIAprep 2.0 Spin Columns“). Das Vakuumverfahren vereinfacht die Handhabung und beschleunigt die Probenverarbeitung. QIAprep 2.0 Spin Columns können mit dem QIAvac 24 Plus oder jedem anderen handelsüblichen Verteiler mit Luer-Anschlüssen vakuumverarbeitet werden. Das QIAprep Spin Miniprep Kit kann nun auch vollautomatisch auf dem QIAcube Connect eingesetzt werden (siehe Abbildung „ QIAcube Connect“).
Das QIAwave Plasmid Miniprep Kit und das QIAprep Spin Miniprep Kit liefern aufgrund ähnlicher chemischer Zusammensetzung die gleiche Leistung. Vergleichsdaten zeigen, dass beide Kits den Kits der Wettbewerber überlegen sind (siehe Abbildungen „QIAwave Kit-Leistung“).
| Format | Spin-Säulen |
| Aufreinigungsmodul | QIAprep 2.0 Spin Columns |
| Durchsatz | 1–24 Proben |
| Vorbereitungszeit | 24 Minipräps in 30 Minuten |
| Erforderliche Arbeitsmittel | Mikrozentrifuge oder Vakuumverteiler; vollständig automatisierbar mit QIAcube Connect |
| Lysatbereinigung | Zentrifugation |
| Fassungsvermögen des Säulenreservoirs | 800 µl |
| Mindestvolumen des Elutionspuffers | 50 µl |
| Kulturvolumen für Plasmide mit hoher Kopienzahl | 1–5 ml |
| Kulturvolumen für Plasmide/Cosmide mit geringer Kopienzahl | 1–10 ml |
Die aufgereinigte DNA kann für den Restriktionsverdau verwendet werden (siehe Abbildung „ Vollständiger Verdau mit verschiedenen Restriktionsenzymen“).
QIAprep 2.0 Spin Columns enthalten eine besondere Silika-Membran, die bei Vorliegen hochkonzentrierter chaotroper Salze bis zu 20 µg DNA bindet und die Elution in einem kleinen Volumen salzarmen Puffers ermöglicht. Mit der QIAprep Membrantechnologie entfallen die zeitaufwändige Phenol-Chloroform-Extraktion und Alkoholausfällung sowie die Probleme und Nachteile, die mit losen Harzen und Aufschlämmungen einhergehen. Die aus den QIAprep 2.0 Spin Columns eluierte hochreine Plasmid-DNA ist sofort einsatzbereit und muss nicht ausgefällt, konzentriert oder entsalzt werden.
Zur schnelleren und komfortableren Bearbeitung und Analyse der Proben enthält das Kit einen Gelladefarbstoff. Der GelPilot Ladefarbstoff enthält 3 Farbstoffe zur Nachverfolgung (Xylencyanol, Bromphenolblau und Orange G), um die Optimierung der Agarosegel-Laufzeit zu erleichtern und zu verhindern, dass kleinere DNA-Fragmente zu weit migrieren (siehe Abbildung „ GelPilot Ladefarbstoff“).
Die Plasmidaufreinigung mit QIAprep Kits und dem QIAwave Plasmid Miniprep Kit erfolgt nach einem einfachen Verfahren mit den Schritten Binden, Waschen und Eluieren (siehe Flussdiagramm „ Das QIAprep Verfahren“). Zunächst werden die Bakterienkulturen lysiert und die Lysate durch Zentrifugation geklärt. Die geklärten Lysate werden dann auf das QIAprep 2.0-Modul aufgebracht, wo die Plasmid-DNA an die Silikamembran adsorbiert. Verunreinigungen werden weggewaschen und die reine DNA wird in einem kleinen Volumen Elutionspuffer oder Wasser eluiert.
Neben der Plasmidaufreinigung aus Escherichia coli können die QIAprep Kits auch zur Aufreinigung von Plasmid-DNA aus Saccharomyces cerevisiae, Bacillus subtilis und Agrobacterium tumefaciens verwendet werden. Hinsichtlich der Protokolle für diese Anwendungen wenden Sie sich bitte an den Technischen Service von QIAGEN oder Ihren Händler vor Ort.
Um Ihre Abläufe zu erleichtern, können Sie Standardprotokolle mit dem TRACKMAN Connected System in Kombination mit PIPETMAN M Connected Pipetten (beide von Gilson) durchführen. Dieses System führt Forschende nicht nur durch die Protokolle, sondern passt auch die Einstellungen der Bluetooth-fähigen Pipetten automatisch an. Weitere Informationen herunterladen.
Das QIAprep Spin und das QIAwave Plasmid Miniprep Kit liefern reproduzierbare Ausbeuten an hochreiner DNA, die sich für die meisten Anwendungen eignet, einschließlich:
Die ACT-Umweltverträglichkeitslabels wurden dafür entwickelt, Produkte nach verschiedenen Nachhaltigkeitskriterien zu bewerten und einzustufen. Die Produkte werden mit 1–10 Punkten bewertet; mit Ausnahme von Energie- und Wasserverbrauch, diemit 1 Punkt pro kWh bzw. Gallone (3,785 Liter) bewertet werden. Je niedriger die Punktzahl, desto geringer die Umweltbelastung. Das QIAwave Plasmid Miniprep Kit (50) hat in den USA einen um 12,4 % niedrigeren Umweltverträglichkeitsfaktor (EIF) als das QIAprep Spin Miniprep Kit (50).
| Features | Specifications |
|---|---|
| Applications | Fluoreszierende und radioaktive Sequenzierung (einschließlich Kapillarsequenzierung), Ligation, Klonierung, Transformation usw. |
| Processing | Manuell (Vakuum oder Zentrifugation) oder automatisiert |
| Plasmid type | High-Copy, Low-Copy, Cosmid-DNA |
| Culture volume/starting material | 1–10 ml Kulturvolumen |
| Elution volume | 50 µl (Minimum) |
| Technology | Silikatechnologie |
| Time per run or prep per run | <30 Minuten |
| Yield | <20 µg |
| Samples per run (throughput) | 1–24 Proben pro Lauf |
| Number of preps per run | 1–24 Proben pro Lauf |