✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
REPLI-g Mitochondrial DNA Kit (25)
Cat. No. / ID: 151023
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Features
- Sensitive amplification from varying sample types
- Suitable for amplification of human and non-human mtDNA
- Overcomes the need for time-consuming isolation of mtDNA
- Informative results from samples with degraded nuclear DNA
- DNA amplification from blood cards and hair
Product Details
The REPLI-g Mitochondrial DNA Kit enables selective amplification of mitochondrial DNA from total DNA samples, without the need for prior mitochondrial DNA isolation. This kit provides DNA polymerase, buffers, and reagents for specific and uniform whole genome amplification from small samples of human and non-human mitochondrial DNA in total DNA samples. For amplification of non-human mtDNA, the REPLI-g Human mt Primer Mix needs to be substituted with an appropriate primer mix suitable for that species (not provided with the kit). The REPLI-g Mitochondrial DNA Kit is based on the multiple displacement amplification technology (MDA), that enriches for mitochondrial DNA with minimal contamination from nuclear DNA, thus avoiding the need for time-consuming isolation of mitochondrial DNA and increasing the sensitivity of downstream assays.
Performance
See figures
Principle
One limitation to the use of mitochondrial DNA has been the need to isolate it from nuclear DNA, particularly in cases where the sensitivity of mitochondrial DNA marker assays needs to be increased. The isolation procedure involves many time-consuming steps and leads to substantial losses of mitochondrial DNA. The REPLI-g Mitochondrial DNA Kit overcomes this limitation by enriching for mitochondrial DNA sequences in total DNA samples.
REPLI-g Mitochondrial DNA technology provides fast and highly uniform DNA amplification across the entire mitochondrial genome. The method is based on multiple displacement amplification (MDA) technology, which carries out isothermal genome amplification utilizing a uniquely processive DNA polymerase. Due to its high processivity and strand displacement activity, REPLI-g DNA Polymerase is capable of amplifying up to 100 kb without dissociating from the DNA template and minimizes unequal sequence and locus representation compared with PCR-based amplification methods.
Procedure
See figures
Applications
REPLI-g amplified mitochondrial DNA can be used in a variety of downstream applications, including:
- Genotyping (e.g., SNP, deletions, insertions)
- Endpoint-PCR, quantitative real-time PCR
- Sequence analysis
Supporting data and figures
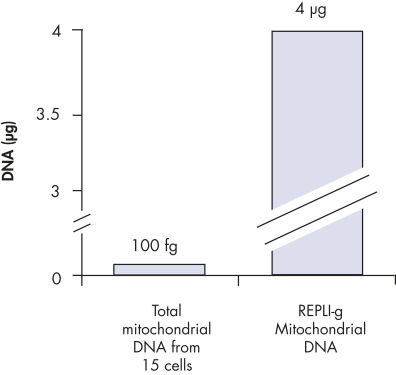
Enrichment of mitochondrial DNA.
Specifications
| Features | Specifications |
|---|---|
| Amplification | Whole genomic DNA |
| Samples per run (throughput) | Mid |
| Denaturation step | Heat |
| Maximum input volume | 10 µl template DNA |
| Minimal pipetting volume needed | 1 µl |
| Reaction volume | 50 µl |
| Reaction time | ~8 hours (overnight) |
| Quality assessment | No |
| Applications | Genotyping, sequencing, RFLP |
| Starting amount of DNA | ~10 ng purified total DNA |
| Starting material | Genomic human DNA |
| Technology | Multiple Displacement Amplification (MDA) |
| Yield | ~4 µg |