Want to try this solution for the first time?
Get in touch with our specialists if you need a quote or if you would like to discuss your project.
PAXgene Blood DNA Tube (IVD) (10 x 100)
Cat. No. / ID: 761165
1000 PAXgene Blood DNA Tubes (IVD) in 10 boxes (100 tubes/box)
The PAXgene Blood DNA Tube (IVD) is intended for in vitro diagnostic use.
Want to try this solution for the first time?
Get in touch with our specialists if you need a quote or if you would like to discuss your project.
Features
- For In Vitro Diagnostic Use
- Compatible with automated purification technologies
- Validated for various collection and storage conditions
- Unique blue cap and label identify tube for DNA testing
- 2D barcode with tube ID, lot number and expiration date
Product Details
The PAXgene Blood DNA Tube (IVD) is a blood collection tube that is robust to handling variability and easily integrated into standard automated workflows.
Performance
The performance of the PAXgene Blood DNA Tube (IVD) has been validated under several processing temperatures and time intervals relevant to variation in blood collection techniques, transport methods and storage conditions. Validation information, along with data from exposure to high temperatures, long-term storage at –20°C and freeze-thaw tests are available under the Resources tab.
The table below summarizes DNA yields from whole blood collected in PAXgene Blood DNA Tubes (IVD) and purified using 2 different technologies. The yield of genomic DNA purified from blood samples collected in PAXgene Blood DNA Tubes (IVD) varies depending on the volume of blood processed, the white blood cell count of the specimen and the selected DNA isolation method.
The table below summarizes DNA yields from whole blood collected in PAXgene Blood DNA Tubes (IVD) and purified using 2 different technologies. The yield of genomic DNA purified from blood samples collected in PAXgene Blood DNA Tubes (IVD) varies depending on the volume of blood processed, the white blood cell count of the specimen and the selected DNA isolation method.
| Extraction method | Number of samples | Input volume (µl) | Output volume (µl) | Lower 95% tolerance limit (µg DNA/ml whole blood) | Median (µg DNA/ml whole blood) | Mean (µg DNA/ml whole blood) |
|---|---|---|---|---|---|---|
| Magnetic beads (QIAsymphony) | 581 | 200 | 200 | 18.2 | 28.9 | 30.2 |
| Silica membrane (QIAcube) | 540 | 200 | 100 | 9.3 | 22.5 | 24.4 |
Principle
The PAXgene Blood DNA Tube (IVD) is a plastic, evacuated blood collection tube designed for in vitro diagnostic DNA testing. The tube includes features that minimize the risk of specimen misidentification: a distinctive blue rubber stopper in the Hemogard closure identifies the tube for DNA testing, and a 2D barcode on the label contains a unique tube identifier number, the tube lot number and the expiration date.
Procedure
Blood is collected into PAXgene Blood DNA Tubes (IVD) using standard phlebotomy techniques. The tube is designed to draw 2.5 ml of whole blood and this volume is easily identified by the fill indicator on the tube label. Venous whole blood can be stored in PAXgene Blood DNA Tubes (IVD) for up to 14 days at room temperature (18–25°C), 28 days at 2–8°C and for up to 52 weeks at –20°C.
For streamlined and standardized processing, PAXgene Blood DNA Tubes (IVD) are designed to integrate into automated workflows. For example, PAXgene Blood DNA Tubes (IVD) are compatible with DNA purification via magnetic beads in combination with QIAsymphony DSP DNA Kits (see figure DNA quality and yield from automated extraction on QIAsymphony). PAXgene Blood DNA Tubes (IVD) are also compatible with DNA purification via silica membrane in combination with QIAamp DSP DNA Blood Mini Kits (see figure DNA quality and yield from automated extraction on QIAcube).
For streamlined and standardized processing, PAXgene Blood DNA Tubes (IVD) are designed to integrate into automated workflows. For example, PAXgene Blood DNA Tubes (IVD) are compatible with DNA purification via magnetic beads in combination with QIAsymphony DSP DNA Kits (see figure DNA quality and yield from automated extraction on QIAsymphony). PAXgene Blood DNA Tubes (IVD) are also compatible with DNA purification via silica membrane in combination with QIAamp DSP DNA Blood Mini Kits (see figure DNA quality and yield from automated extraction on QIAcube).
See figures
Applications
PAXgene Blood DNA Tubes (IVD) can be used for molecular diagnostic methods requiring DNA.
Supporting data and figures
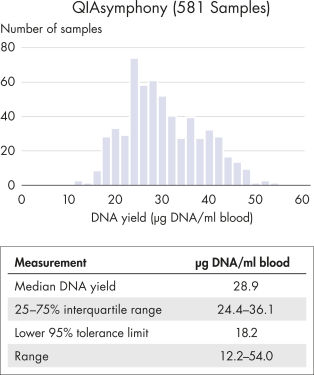
DNA quality and yield from automated extraction on the QIAsymphony.
Samples collected in PAXgene Blood DNA Tubes (IVD) were processed at one site (internal) on the QIAsymphony, which performs automated DNA purification based on magnetic bead extraction. Median DNA yield was 28.9 µg DNA/ml blood, with 95% of samples ≥18.2 µg DNA/ml blood. DNA purity is equally high, with an A260/A280 ratio of 1.7–1.9. Extraction was performed using QIAsymphony DSP DNA Kits (200 µl blood sample input, 200 µl DNA eluate Output).
Resources
Kit Handbooks (1)
Safety Data Sheets (2)
White Papers (1)
Brochures & Guides (1)
Application Notes (1)
FAQ
How should blood samples drawn into PAXgene Blood DNA Tubes (IVD) be stored?
Where can I find additional information for PreAnalytiX PAXgene products?
Do I have to use a blood collection set with PAXgene Blood DNA Tubes (IVD)?
What is the shelf life of PAXgene Blood DNA Tubes (IVD) prior to blood collection?
Are there any special considerations for the collection of blood using PAXgene Blood DNA Tubes (IVD) (e.g., use of a blood collection set or discard tube, positioning of the tube, issues with short draws, etc.)?
How should blood samples drawn into PAXgene Blood DNA Tubes (IVD) be transported?
How long can samples in PAXgene Blood DNA Tubes (IVD) be stored at room temperature?
How long can samples in PAXgene Blood DNA Tubes (IVD) be stored at 2–8°C?
What is the expected purity of DNA extracted from blood samples collected in PAXgene Blood DNA Tubes (IVD)?
How should frozen PAXgene Blood DNA Tubes (IVD) be thawed?
Can PAXgene Blood DNA Tubes (IVD) be stored at higher than room temperature (18–25°C)?
What is the additive in PAXgene Blood DNA Tubes (IVD)?
Which downstream applications have been tested with DNA purified from samples collected in PAXgene Blood DNA Tubes (IVD)?
What is the expected DNA yield from 2.5 ml of blood collected in the PAXgene Blood DNA Tube (IVD)?
Are PAXgene Blood DNA Tubes (IVD) sterile?
Can blood samples be frozen in PAXgene Blood DNA Tubes (IVD)?



