PyroMark Q24 MDx
使用焦磷酸测序技术进行IVD验证的突变和甲基化分析
使用焦磷酸测序技术进行IVD验证的突变和甲基化分析
PyroMark Q24 MDx实时定量焦磷酸序列分析仪应用经验证的焦磷酸测序技术,进行基于实时序列的定量检测分析,在欧洲和中国用于体外诊断。这个创新的平台非常适合用于突变的基因分型、评估疾病相关的DNA甲基化模式、验证生物标记物以及其他诊断相关的分析。
焦磷酸测序技术可对遗传学和表观遗传学的DNA变化进行准确、灵敏的定量分析,提供高度可靠的序列数据。可鉴定新的突变,并检测低水平的DNA甲基化模式异常。
步骤1:扩增DNA片段,作为焦磷酸测序模板的单链是生物素标记的。变性后,分离生物素标记的单链PCR扩增子,与测序引物杂交。杂交的引物和单链模板同各种酶,包括DNA聚合酶、ATP硫酸化酶、荧光素酶、三磷酸腺苷双磷酸酶以及底物、腺苷酰硫酸(APS)和荧光素一起孵育(参见"Principle of Pyrosequencing — step 1")。
步骤2:第一个脱氧核苷三磷酸(dNTP)加入反应中,在DNA聚合酶的作用下,dNTP结合到DNA链上与其互补的碱基位。每次结合都会释放与核苷酸相等摩尔量的焦磷酸脂(PPi)(参见"Principle of Pyrosequencing — step 2")。
步骤3:在存在APS的条件下,ATP硫酸化酶将PPi转化为ATP。在这个ATP的驱动下,荧光素酶将荧光素转化为氧化荧光素,并产生可见光,可见光的量与ATP的量成正比。通过电荷耦合(CCD)相机即可检测到荧光素酶催化反应产生的可见光,并在原始数据输出(Pyrogram)中显示为一个峰,每个峰的高度(光信号)与结合的核苷酸数量成正比(参见"Principle of Pyrosequencing — step 3")。
步骤4:三磷酸腺苷双磷酸酶,是一种核苷酸降解酶,会降解未结合的核苷酸与ATP。完全降解后,加入另一个核苷酸(参见"Principle of Pyrosequencing — step 4")。
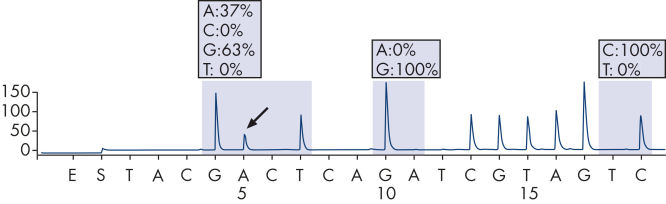
步骤5:持续添加dNTP。α-硫代脱氧腺苷三磷酸(dATPαS)在反应中用于替代天然脱氧腺苷三磷酸(dATP),这是因为DNA聚合酶可高效的应用前者,而不被荧光素酶识别。当该过程连续进行时,就会形成互补的DNA链,可根据Pyrogram追踪的信号峰确定核苷酸的序列(参见"Principle of Pyrosequencing — step 5")。
QIAGEN提供与PyroMark Q24 MDx实时定量焦磷酸序列分析仪配套的IVD验证分析。目前,这些试剂盒包括一些重要的癌症相关突变。
| 产品 | 用于定量检测的突变 |
|---|---|
| therascreen KRAS Pyro Kit | 人KRAS基因密码子12、13和61 |
| therascreen BRAF Pyro Kit | 人BRAF基因密码子600和464-469 |
| therascreen EGFR Pyro Kit | 人EGFR基因密码子719、768、790、858和861以及外显子19 |
| therascreen NRAS Pyro Kit | 人NRAS基因密码子12、13和61 |
从PCR产物到直接用于测序的单链模板,应用PyroMark Q24 MDx Vacuum Workstation可在15分钟内同时制备24个样本。该工作站操作简单,且手动时间少于5分钟。
在焦磷酸测序之前,形成了一个生物素标记的PCR产物,该产物结合到覆盖链霉亲和素的琼脂糖凝胶珠上。这些胶珠会被真空仪器捕捉并彻底清洗,随后变性,产生适合于焦磷酸测序的单链DNA。该模板DNA放入含测序引物的焦磷酸测序反应板中,随后引物退火,将该板放入PyroMark仪器中。PyroMark Gold试剂包含用于焦磷酸测序反应的酶、核苷酸和底物,将这些试剂移入配药管或筒(取决于仪器),按照软件中提供的量放入仪器中,用于焦磷酸测序。
PyroMark Q24 MDx实时定量焦磷酸序列分析仪适合体外诊断应用,用于检测与临床相关的特定可变DNA位点的变化。
PyroMark Q24 MDx Software装载在PC机上,能够全面的分析您的结果。该软件包含2个分析模块:CpG和AQ(等位定量)。2个模块可分析在同一孔板中的样品,不同类型的样品可同时分析。AQ模块用于分析单个和di-、tri-和tetra-多个等位突变。CpG模块用于分析多个连续的CpG位点并提供经亚硫酸氢盐转化的内参。
| Features | Specifications |
|---|---|
| Connections | One USB port (2.0) |
| Chemical resistance | pH 4 to pH 9, common detergents, 0.5 M sodium hydroxide, ethanol |
| Applications | Methylation analysis, allele quantification, genotyping, sequence analysis |
| Humidity | Relative humidity of 20–90% (noncondensing) |
| CE/FDA/IVD compatible | In Europe |
| Instrument dimensions | 420 x 390 x 525 mm (16.5 x 15.4 x 20.7 in.) |
| Kits designed for this instrument | IVD-labeled therascreen Kits |
| Operating temperature | 15–32°C (59–90°F) |
| Overvoltage category | II |
| Place of operation | For indoor use only |
| Pollution level | 2 |
| Power | 100–240 V AC, 47–63 Hz, 1.1–0.45 A (grounded). From external power supply to instrument: 12 VDC and 24 VDC nominal |
| Process temperature | 28°C (82.4°F) ± 1°C |
| Process time | Depends on the number of nucleotide dispensations (20 dispensations take 24 minutes) |
| Samples per run (throughput) | 1–24 |
| Software | PyroMark Q24 MDx Software 2.0 |
| Technology | Pyrosequencing |
| Weight | 27.5 kg (60.6 lb) |
| Altitude | Up to 2000 m (6500 ft) |